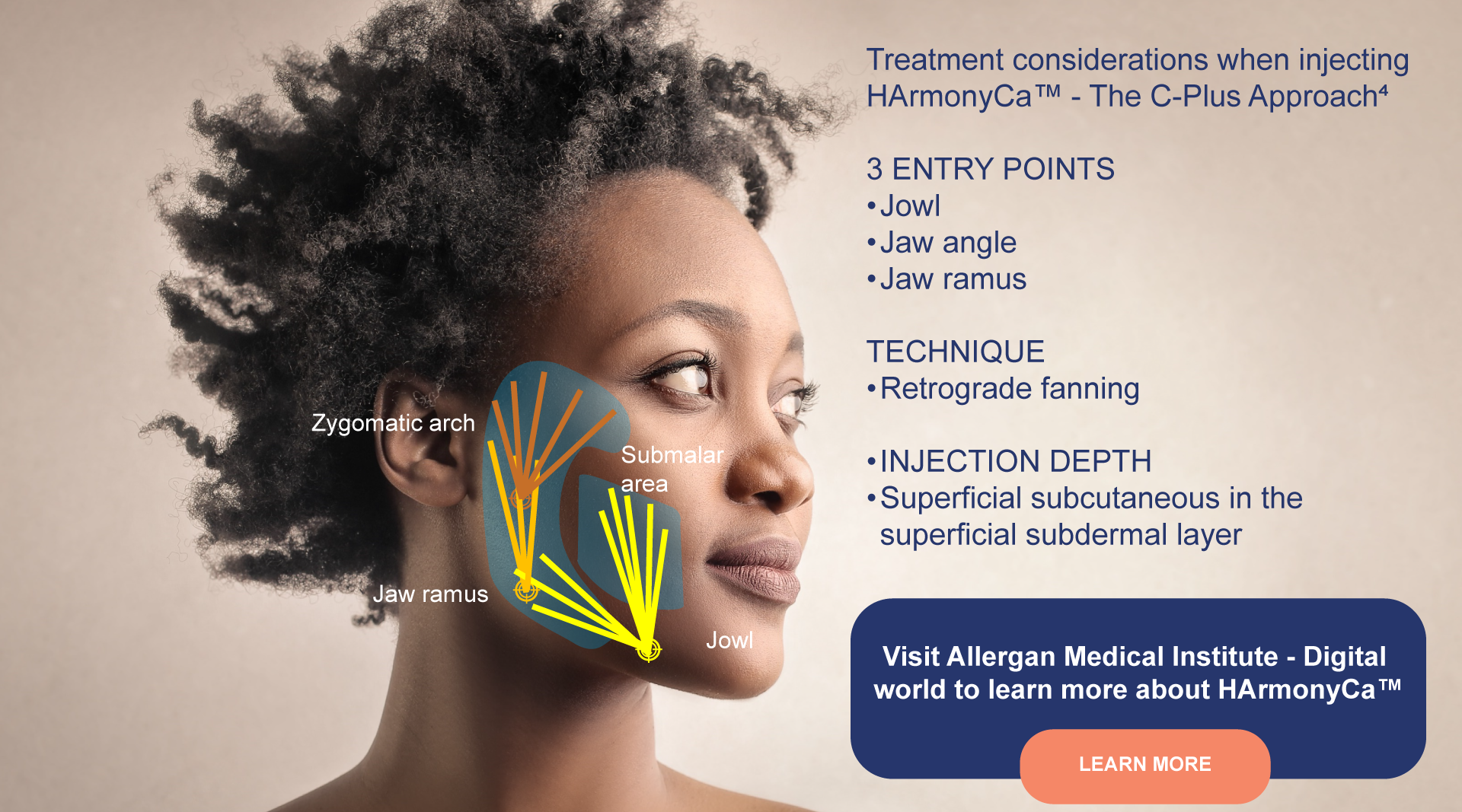
Deliver HA and CaHA in one single
injection with HArmonyCa™¹ |
Immediate and sustained
lifting effect2-3 |
|
Rejuvenates the collagen
production2-3 |
|
Restores elasticity & firmness
that builds over time for
youthful-looking skin2-3 |
|
|
|
References:
1. HArmonyCa™ Lidocaine IFU. M032 V01. 2021. 2. Urdiales-Gálvez F et al. J Cosmet Dermatol. 2023;00:1-12. 3. Bravo BSF et al. Plast Reconstr Surg Glob
Open. 2023 June15: 11(6). 4. Braz A et al. A Novel Hybrid Injectable for Soft-tissue Augmentation: Analysis of Data and Practical Experience. Plastic & Reconstructive
Surgery-Global Open 12(9):p e6190, September 2024 |
If you would like to contact us, please mail all communications to: AbbVie AB | Box 1523, 171 29 Solna | +46 (0)8 684 44 600 | info@abbvie.se. Abbvie reserves
the right to alter or cancel the program with no advance notice or obligation. © 2022 AbbVie. All rights reserved. All trademarks are the property of their respective
owners. Approval number SE-AGNA-250001 | Date of preparation: January 2025. |
| |
|
|
HArmonyCa™
HArmonyCa™ Lidocaine is referred to as HArmonyCa™. HArmonyCa™ is a dermal filler intended for facial soft tissue augmentation and should be injected into the deep dermal and sub-dermal layers. HArmonyCa
™ injection may be accompanied with mild discomfort; administration of anesthetics should be considered. As with all transcutaneous procedures, injection of HArmonyCa™ carries a risk of infection. To reduce
this risk, common practice of such procedures should be followed. If nodules appear, patient should massage the treated area. Patient should be informed that the injected material may be palpable for a long
period after treatment. Common postoperative adverse events include erythema, edema (swelling), pain, tenderness, and itching. Treatment site reactions typically resolve within 24-48 hours and swelling within a
week. Less common adverse events associated with dermal fillers in general and calcium hydroxyapatite-based fillers in particular include hematoma, seroma, extrusion, induration, skin pigmentation, fistula
formation, inflammatory reaction, infection, allergic reaction, migration, persistent nodules, granulomas, necrosis and blindness. See IFU for a full list of contraindications, precautions and undesirable effects.
HArmonyCa™ is a medical device Class III CE2409. ©2022 Allergan Aesthetics, an AbbVie Company. All rights reserved. All trademarks are the property of their respective owners. |