|
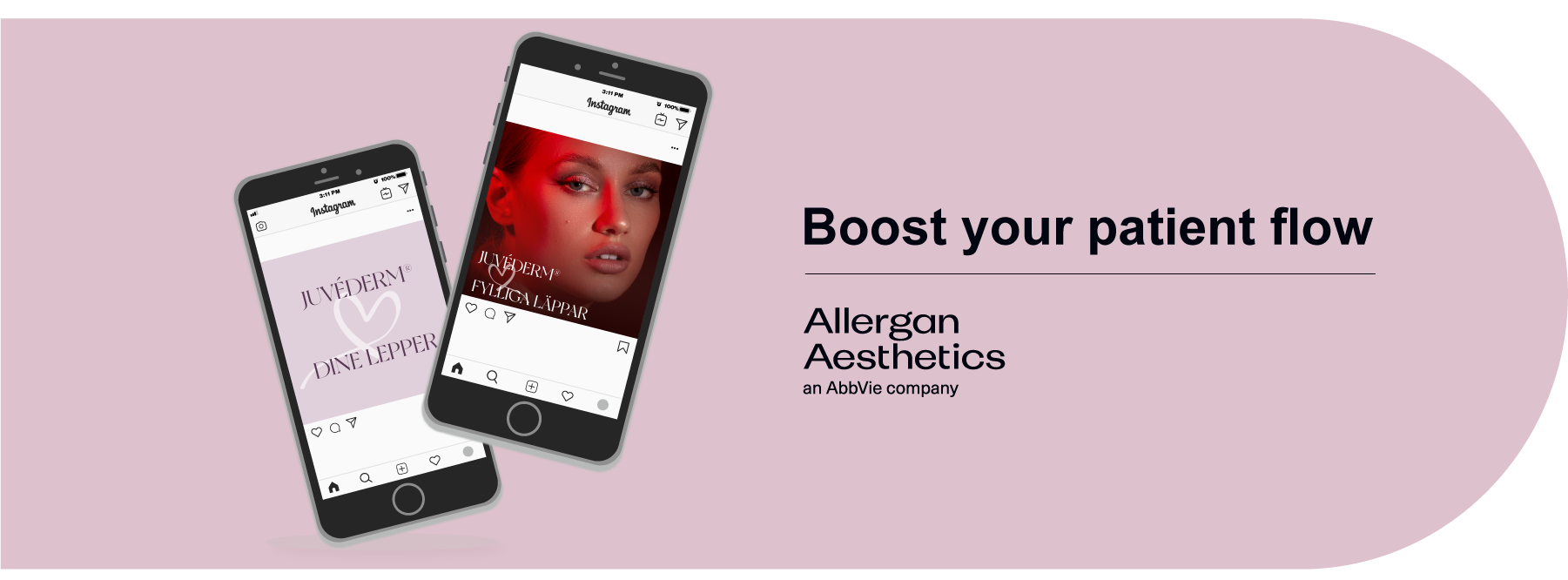
| Boost your clinics patient flow with available campaign materials for Social Media |
| These materials are available in Norway Just click the download button and yor are on your way! If you are curious on other available market materials you can find these HERE |
|
|
Download the Implementation guide for LIFT UP SoMe posts HERE |
|
|
|
|
| Juvéderm® JUVÈDERM® Material provided by Allergan Aesthetics, an AbbVie company. AbbVie AB | Box 1523, 171 29 Solna | +46 (0)8 684 44 600 | info@abbvie.se. All rights reserved. All trademarks are the property of their respective owners. JUVÈDERM® is a medical device Class III CE0334. License details and indications may vary between countries - please consult individual product ”Directions for Use”. The most commonly reported side effects with JUVÉDERM® injectable gels included redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. www.juvederm.se www.juvederm.dk www.juvederm.fi www.juvedermit.no HArmonyCa™ HArmonyCa™ Lidocaine is referred to as HArmonyCa™. HArmonyCa™ is a dermal filler intended for facial soft tissue augmentation and should be injected into the deep dermal and sub-dermal layers. HArmonyCa ™ injection may be accompanied with mild discomfort; administration of anesthetics should be considered. As with all transcutaneous procedures, injection of HArmonyCa™ carries a risk of infection. To reduce this risk, common practice of such procedures should be followed. If nodules appear, patient should massage the treated area. Patient should be informed that the injected material may be palpable for a long period after treatment. Common postoperative adverse events include erythema, edema (swelling), pain, tenderness, and itching. Treatment site reactions typically resolve within 24-48 hours and swelling within a week. Less common adverse events associated with dermal fillers in general and calcium hydroxyapatite-based fillers in particular include hematoma, seroma, extrusion, induration, skin pigmentation, fistula formation, inflammatory reaction, infection, allergic reaction, migration, persistent nodules, granulomas, necrosis and blindness. See IFU for a full list of contraindications, precautions and undesirable effects. HArmonyCa™ is a medical device Class III CE2409. ©2022 Allergan Aesthetics, an AbbVie Company. All rights reserved. All trademarks are the property of their respective owners. |