|
| This information is intended for healthcare professionals only |
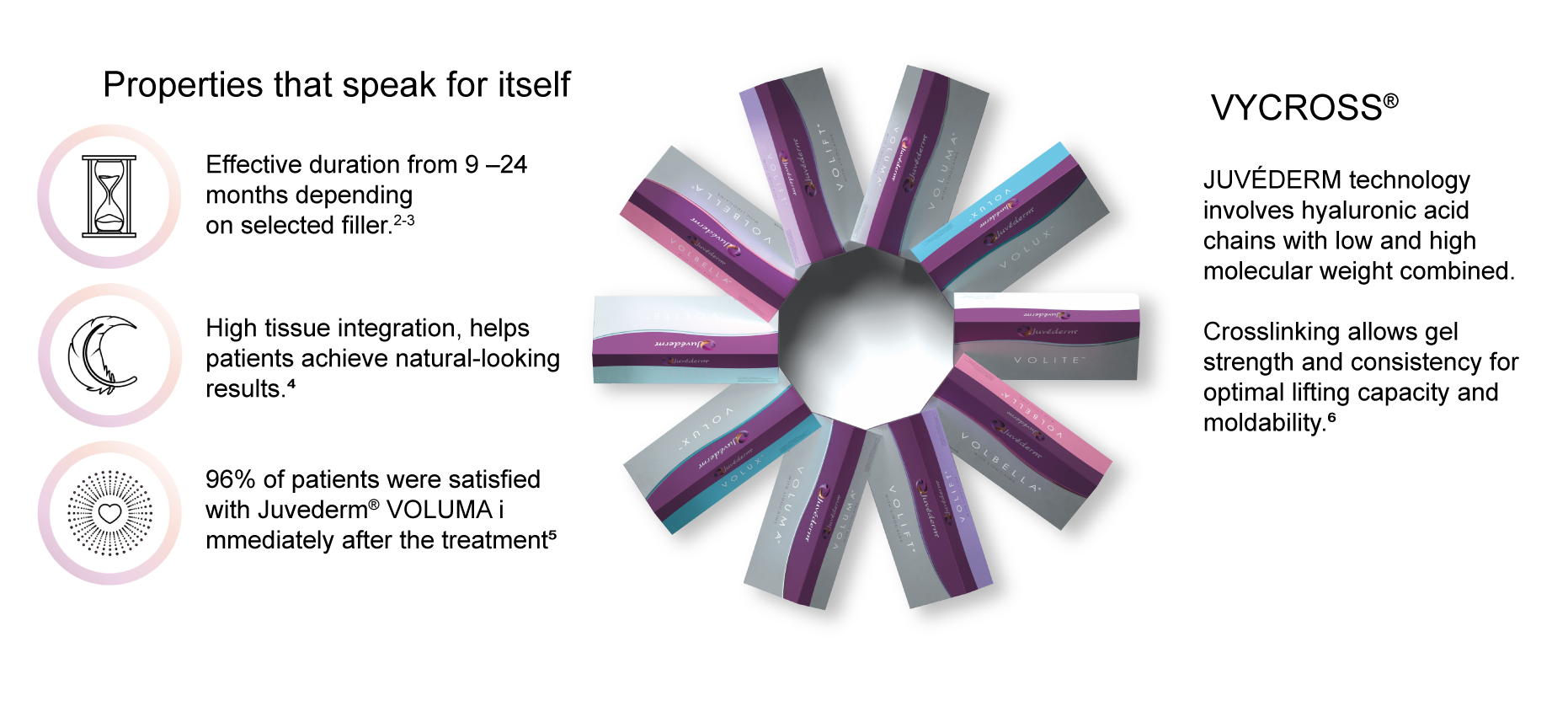
JUVÉDERM® –Long Effectiveness, natural results & high patient satisfaction |
|
|
|
Individualised result with VYCROSS® |
|
| References: 1. Liew S et al. J Cosmet Dermatol. 2020;19:296–302 2. Pinsky MA et al. Aesthetic Surg J. 2008;28:17–23. 3. Callan P et al. Clin Cosmet Invest Dermatol. 2013; 681-89. 4. Hee CK et al. Dermatol Surg. 2015;41(Suppl 1):S373–381. 5. Beer K et al. Dermatol Surg. 2021;47:80–85. 6. de la Guardia, C., Virno, A., Musumeci, M., Bernardin, A., & Silberberg, M. B. (2022). Facial plastic surgery : FPS, 10.1055/s-0041-1741560; https://doi.org/10.1055/s-0041-1741560 |
|
|
|
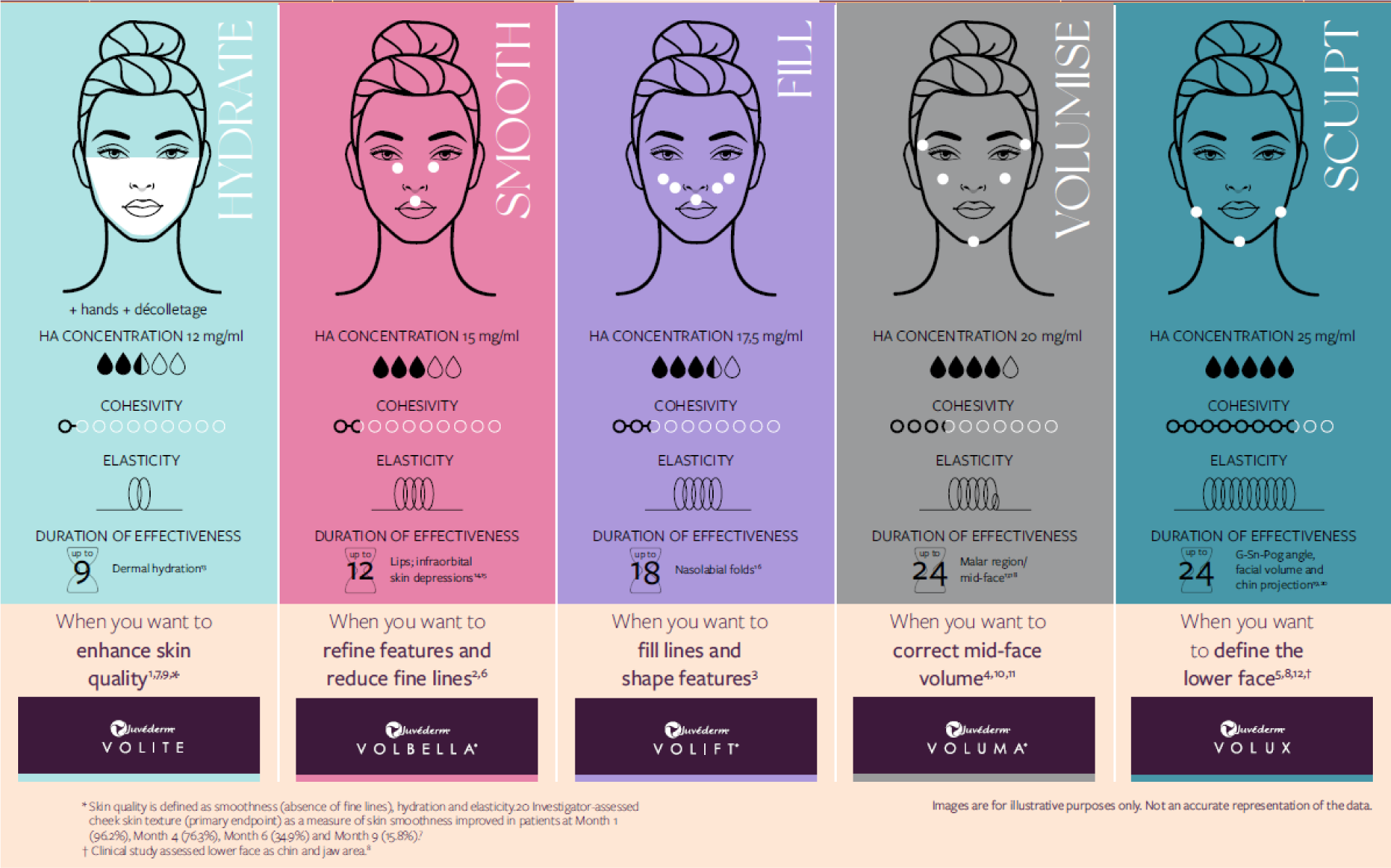
Embracing Individuality¹ for you and your patients |
|
| References: 1. Liew S et al. J Cosmet Dermatol. 2020;19:296–302 2. Baumann LS et al. Dermatol Surg. 2007;33:S128–35 3. Goodman GJ et al. Plast Reconstr Surg. 2015;136:139S–48S. 4. Pinsky MA et al. Aesthetic Surg J. 2008;28:17–23.5. Callan P et al. Clin Cosmet Invest Dermatol. 2013; 681-89. 6. de la Guardia, C., Virno, A., Musumeci, M., Bernardin, A., & Silberberg, M. B. (2022). Facial plastic surgery : FPS, 10.1055/s-0041-1741560; https://doi.org/10.1055/s-0041-1741560. 7. Juvéderm ULTRA DFU. |
|
|
|
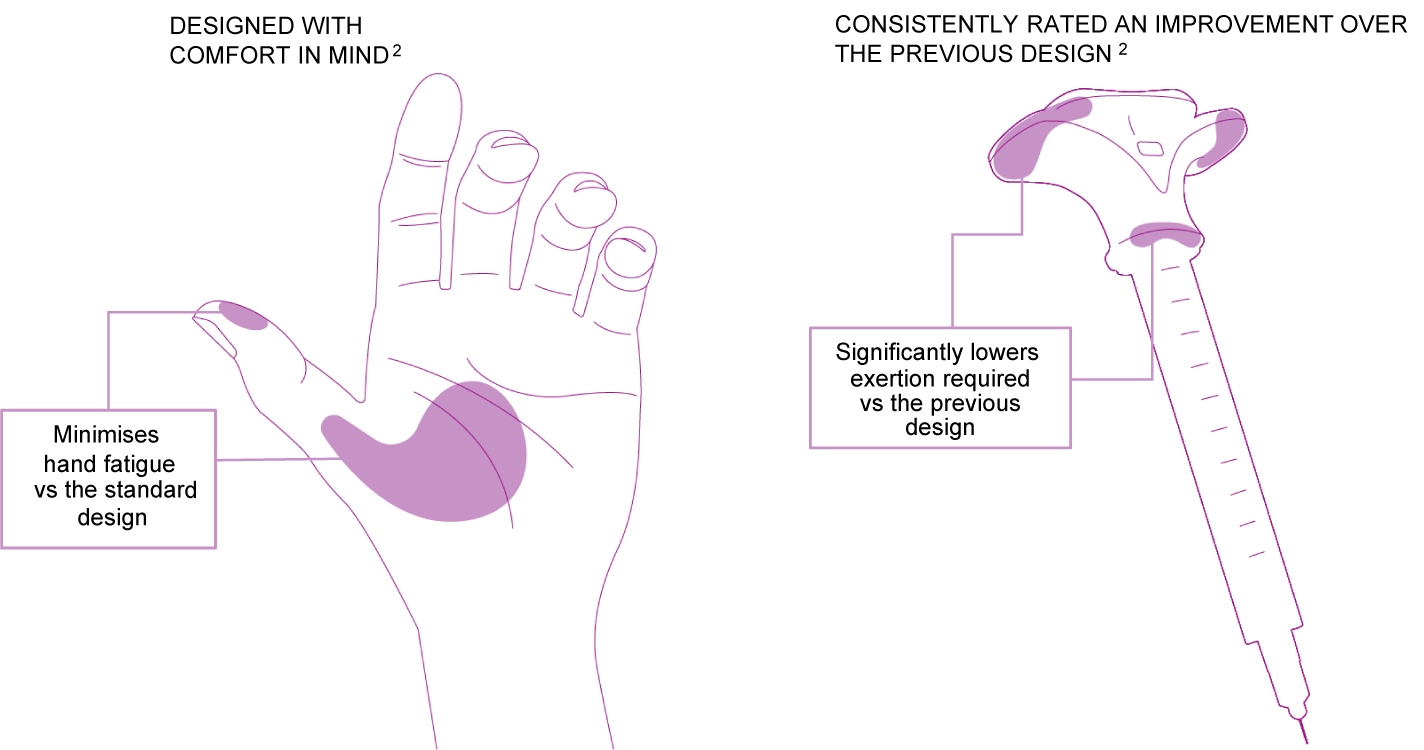
Enhanced Injection Experience with JUVEDERM® |
Improved ease, comfort and muscle fatigue while injecting via JUVÉDERM®’s innovative ergonomic syringe1,2 |
|
|
|
|
|
| References: 1. Costello K et al. In-field assessment of the perception of smoothness when dispensing different filler formulations. International Master Course on Aging Science (IMCAS), 3–5 June 2022, Paris, France. https://abbvie1.outsystemsenterprise.com/GMAEventPublications/Assets. aspx?ConferenceId=299 2. Costello K et al. A new dermal filler syringe with improved ergonomic performance to enhance end-user experience. International Master Course on Aging Science (IMCAS), 3–5 June 2022, Paris, France. https://abbvie1.outsystemsenterprise.com/GMAEventPublications/Assets. aspx?ConferenceId=299. |
|
|
|
| Juvéderm® JUVÈDERM® Material provided by Allergan Aesthetics, an AbbVie company. AbbVie AB | Box 1523, 171 29 Solna | +46 (0)8 684 44 600 | info@abbvie.se. All rights reserved. All trademarks are the property of their respective owners. JUVÈDERM® is a medical device Class III CE0334. License details and indications may vary between countries - please consult individual product ”Directions for Use”. The most commonly reported side effects with JUVÉDERM® injectable gels included redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. www.juvederm.se www.juvederm.dk www.juvederm.fi www.juvedermit.no |